T-PDT Phototherapy treatment
The system FLUXMEDICARE® V2 is a Class IIa medical device in accordance with Directive 93/42 EEC manufactured by MDBTexinov®, and the conformity assessment for which was performed by the BSI organisation.
The accessories are CE marked Class I medical devices in accordance with Regulation (EU) 2017/745.
Class/Notified body: IIa / CE 2797
Manufacturer: MDB Texinov®
It is intended for health professionals in the context of treatment for certain dermatological conditions (thin, non-pigmented actinic keratoses of OLSEN Grade 1 and 2).
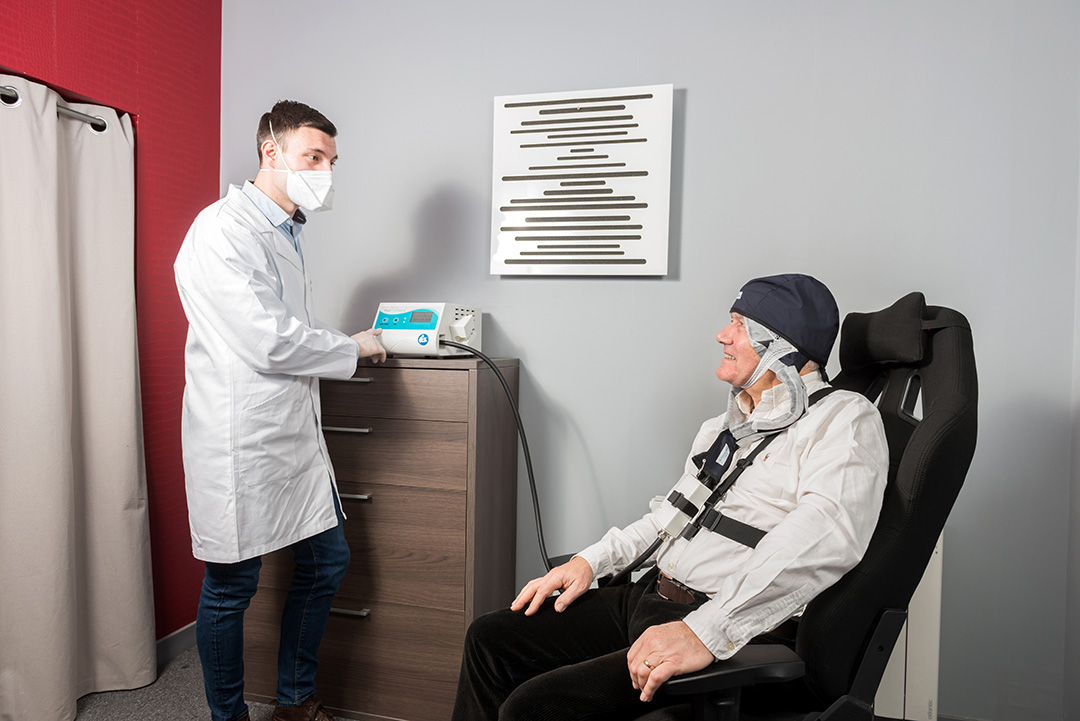
- Cream incubation time: 30 min (3 h for C-PDT)
- Exposure phase: 2 h 30 min (30 min for C-PDT).
- Total saving: 30 min
Can be used throughout the year: the treatment is not dependent on daylight or outside weather conditions (bad weather, heat wave).
* To use this product correctly it is recommended that the indications and contraindications listed in the followed instructions for use:
The instructions for the previous version of the device are available by following this link.




