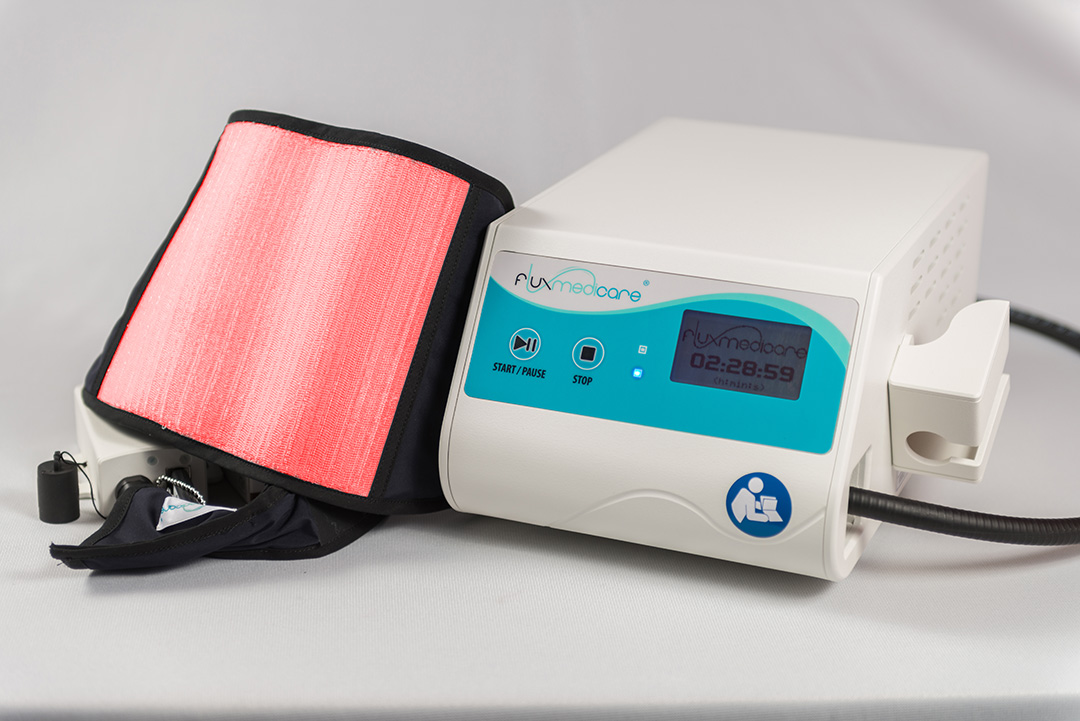
PDT solution dedicated to the treatment of actinic keratoses
Photodynamic Therapy differently with Textile-PDT


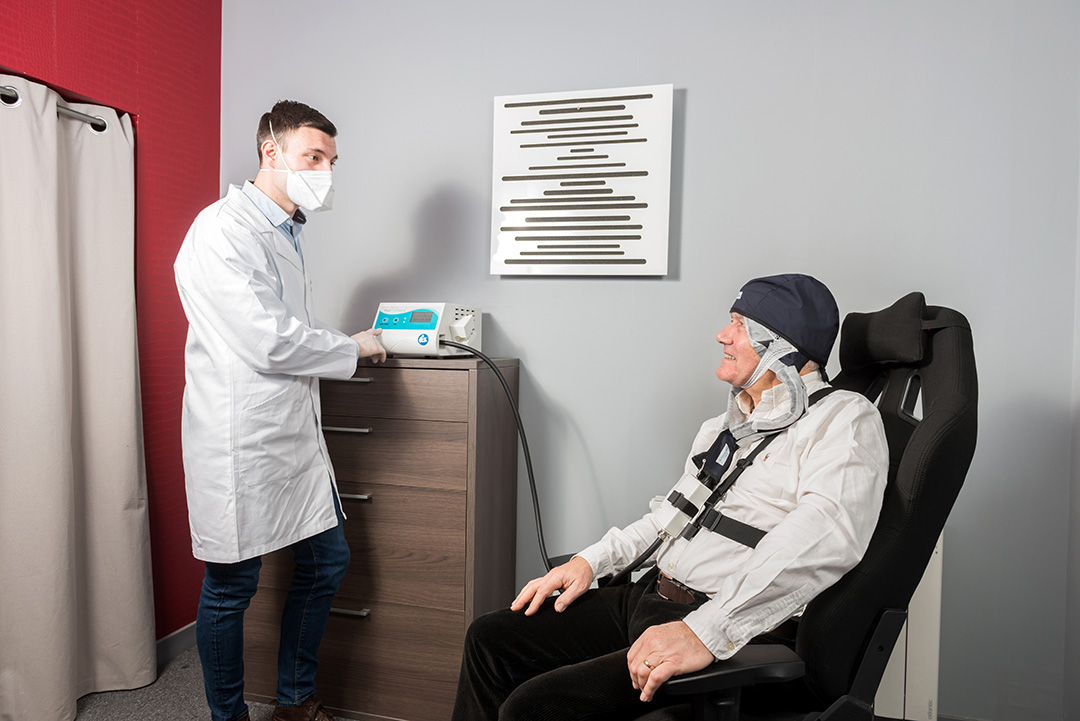
Almost painless for the patient


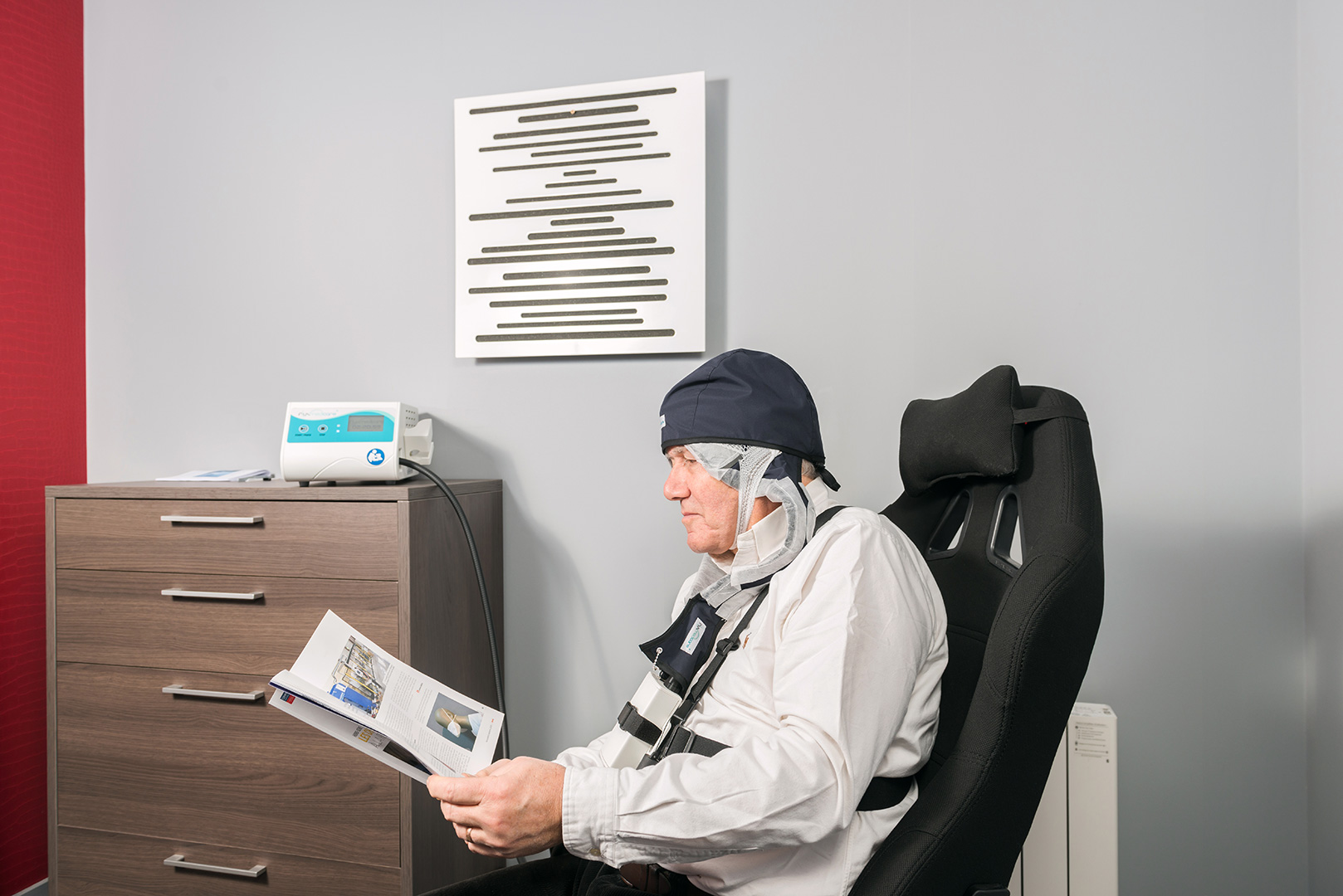
Patient comfort and mobility


Results equivalent to C-PDT


Ease of use for the practitioner
“THE MAGIC CAP
called Fluxmedicare®
The advantages: there is no burning or heat sensation during the treatment. In addition, because the soft fabric adapts flexibly, the patient can move relatively freely and, for example, read at the same time. Protective equipment, cooling or anesthesia are not necessary, and thanks to the light diffusion technology, a completely homogeneous and targeted treatment of the damaged skin areas is possible.”


Chief Medical Officer Prof. Dr. Rolf-Markus Szeimies
KLINIKUM VEST – Dermatology and Allergy Department (Germany)
Exclusive Textile-PDT light diffusion
Dedicated
to dermatology
PDT
in Practice

News
Interview with the Head of the Dermatology and Allergology department of Klinikum Vest (Germany)
The advantages of the Fluxmedicare® device highlighted through an interview conducted by our German distributor with rof. Dr. Rolf-Markus Szeimies, Head of the Dermatology and Allergology Department at Klinikum Vest (germany). Read the interview (in German)
Publication by Prof. Szeimie in Klinikum Vest review
Article retracing the advantages of FLUXMEDICARE® in the Phototherapy environment compared to other devices for the treatment of pre-cancers of the skin. Read the article (in German)
A patented fibre optic luminous textile that treats precancerous skin lesions
Resulting from several years of research in collaboration with reference laboratories and doctors, the Fluxmedicare device designed and manufactured in Isère by MDB Texinov revolutionises the conditions for treatment of skin diseases by photodynamic therapy (PDT) by...
Events
No events organised at the moment.
Don’t hesitate to visit the website frequently for updates.
Thank you.